Examine the Redox and Nonredox Reactions in Model 1
Metal Nonmetal Compound 2. This is the currently selected item.

Ruben Oxidation And Reduction S Pdf Ruben Macias Nova Oxidation And Reduction What Happens When Electrons Are Transferred In A Chemical Course Hero
Double Replacement two switch.

. This makes sense since as one reactant is losing electrons being oxidized the other is gaining electrons being reduced Oxidation numbers can be helpful in determining whether a reaction is redox. How does one recognize a redox reaction. O atoms are balanced M n O 4 8 H M n 2 4 H 2 O.
Here is one of the simplest examples of these reactions that will help you to get a better idea of this concept. - Redox portmanteau of reduction and oxidation reactions include all chemical reactions in which atoms have their oxidation state changed. A redox reaction can easily be explained as.
Isare there any features in the redox reactions that would allow you to identify them as redox reactions. Balancing a redox equation in basic solution. Isare there any features in the redox reactions that would allow you to identify them as redox.
What two types of reactions are shown in Model 1. In the space under each reaction in the model write the oxidation number for every atom. In activity 17 water is electrolysed to give H 2 gas at one electrode and O 2 gas at the other electrode according to the given chemical reaction.
Metal Hydroxide Metal bonded with OH Redox SynthesisComposition Element Element Compound 1. Balance other than O and H M n O 4 M n 2 4 H 2 O. Up to 24 cash back 9.
Determine the oxidation numbers for ALL species in reactions A-D. Isare there any features in the redox reactions that would allow you to identify them as redox reactions. Nonmetal Hydrogen.
Solved 3 In The Space Under Each Reaction In Model 1 Write Chegg Com NoellegroCampbell Home. What is the concentration of NaClaq. 1 The atom in its elemental form has an oxidation number of zero.
A redox reaction is a reaction in which a transfer of electrons occurs from one reactant to another. Decrease in oxidation state. Solved 3 In The Space Under.
Single Replacement one replaces another. Mg Mg2 2e D. If yes use specific examples from Model 1.
What happens in a reduction. 2 Monatomic ions have an oxidation number equal to. In the reaction MgCl2MgCl2 the correct half-reaction for the oxidation that occurs is A.
If yes use specific examples from Model 1 to support your answer. A reduction and oxidation reaction. No we cant identify redox rxns by.
Forming a bond by sharing electrons. Isare there any features in the redox reactions that would allow you to identify them as redox reactions. An example is shown here.
In the space under each reaction in Model 1 write the oxidation number for every atom. Isare there any features in the redox reactions that would allow you to identify them as redox reactions. Examine the redox and nonredox reactions in Model 1.
An example is shown. Include specific examples if so. Problems on Balancing Redox Reactions.
M n O 4 M n 2. The breakdown of glucose in cells. The temporary storage of.
These include Baby Mario Light Mario Medium Luigi and Heavy Luigi. The oxidized element increases in oxidation number while the reduced element decreases in oxidation number. 2H 2 Ol 2H 2 g O 2 g Thus two molecules of water on electrolysis give 2 molecules of hydrogen gas and one molecule of oxygen gas or the amount of hydrogen gas collected would be double.
Cl2 2e 2Cl C. Oxidizing and reducing agents. If yes use specific examples from the model to support your answer.
Examine the redox and nonredox reactions in Model 1. Are there any features of the redox reactions that would allow you to identify them as redox reactions. Mario Super Sluggers Super Mario Wiki.
A redox reaction must involve a change in oxidation number for two of the elements involved in the reaction. Balancing a redox equation in acidic solution. Identify the following reactions as either redox or nonredox.
Home Reactions Examine the Redox and Nonredox Reactions in Model 1 Examine the Redox and Nonredox Reactions in Model 1 sa_Darren758 April 22 2022. Redox states are determined from a set of rules listed below. H 2 F 2 2HF.
Single-replacement reactions are redox reactions because two different elements appear as free. Up to 24 cash back 1. The reaction that takes place in a chemical cell is best classi ed as A.
Formation of Hydrogen Fluoride. Compare and contrast the two types of reactions included in Model 1. An element gains one or more electrons.
If yes use specific examples from Model 1 to support your answer. Examine the redox and nonredox reactions in Model 1. Isare there any features in the redox reactions that would allow you to identify them as redox reactions.
Balance the following redox reaction in the acid medium by the ion-electron method. - The term redox comes from two concepts involved with electron transfer. The four sets of chemical reactions shown in Model 1 have the following general names.
What two types of reactions are shown in Model 1. Redox reactions are classified by having both an oxidation reaction and a reduction reaction and hence an oxidizing agent and a reducing agent. Write a net ionic equation for this reaction.
What two types of reactions are shown in Model 1. Redox and Nonredox Reactions. Examine the redox and nonredox reactions in Model l.
F 2 2e. Up to 24 cash back In the reaction MnO 4-aq oxidizes the chloride ions to chlorine while the manganese is reduced to Mn2aq. Oxidation and Reduction 1 3.
Omg i lyke hate mi lyfe dont 4get 2 balance those equations lol. Divide the work among your group members. In the redox reaction AgNO3Na -- NaNO3 Ag how many electrons were transferred.
Which equation represents the half-reaction that takes place at. Discuss within your group which name belongs to which set of chemical reactions. Examine the redox and non-redox reactions.
After you have done so then go to the waterfall. In general redox reactions involve the transfer of electrons between species. Student who could assign ox on previous WS were confused by.
Which of the following most completely describes a redox reaction. Conversely in a nonredox reaction no electron transfer occurs. If yes use specific examples from Model 1.
H 2 2H 2e. A 1000 mL sample of NaClaq is reacted with 210 mL of 130 molL KMnO 4aq. Examine the redox and nonredox reactions in Model 1.

35 Oxidation And Reduction S 35 Oxidation And Reduction S 35 Oxidation And Reduction S Oxidation Studocu
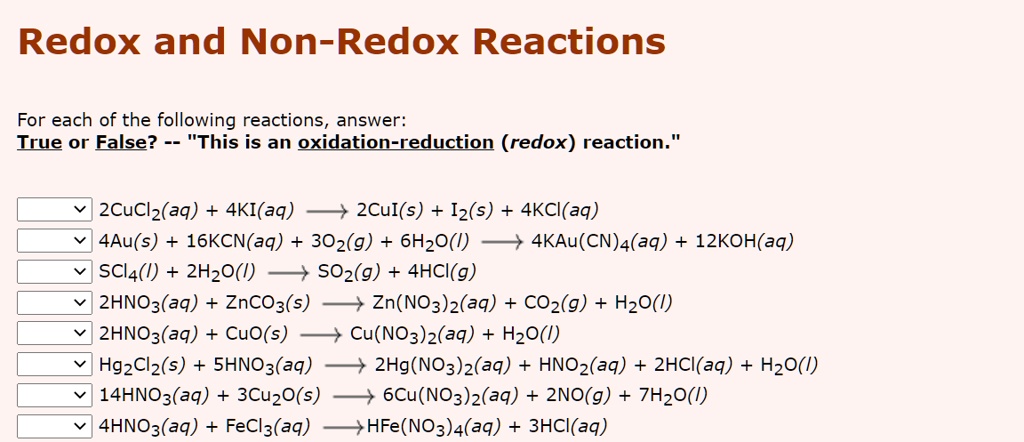
Solved Redox And Non Redox Reactions For Each Of The Following Reactions Answer Irue Or False This Is An Oxidation Reduction Redox Reaction 2cuclz Aq 4ki Aq 2cul S Iz S 4kci Aq 4au S 16kcn Aq 302 G 6h2o 4kau Cn A Aq

Solved 3 In The Space Under Each Reaction In Model 1 Write Chegg Com

No comments for "Examine the Redox and Nonredox Reactions in Model 1"
Post a Comment